You are here
Journal Club for February 2024: Mechanics in Solid-State Batteries: Mechanical Properties, Interfacial Failure, and Multiphysics Modeling
Journal Club for February 2024: Mechanics in Solid-State Batteries: Mechanical Properties, Interfacial Failure, and Multiphysics Modeling
Wei Li=, Ruqing Fang=, Junning Jiao, Juner Zhu*
Department of Mechanical and Industrial Engineering, Northeastern University
* Corresponding author: j.zhu@northeastern.edu
= Authors with equal contributions to this article
1. General introduction
Exciting new challenges are posted to the community of mechanics as our real-world engineered systems get increasingly complex. One of them is the deep coupling between mechanical principles and other physical effects. In this month’s iMechanica Journal Club, we would like to introduce everyone to some interesting mechanics-related problems in a type of advanced engineered system, namely solid-state batteries (SSBs). SSBs are often referred to as a “next-generation battery.” There are two folds of implications behind this name. On the one hand, they are considered a promising alternative to conventional Li-ion batteries thanks to their enhanced safety and increased energy density. On the other hand, there are still many unsettled technical bottlenecks to be overcome before their commercialization. It is worth noting that as of the time this journal club blog is written, SSBs have not been successfully applied to any commercial electric vehicle models.
Compared to their liquid-electrolyte counterparts, SSBs have a similar set of governing physics, including electrochemistry, mechanics, thermal behavior, etc. Several important topics about liquid-electrolyte Li-ion batteries have been comprehensively overviewed by previous iMechanica Journal Club articles:
December 2020: 3D Printing of Batteries: Fabrication, Materials and Challenges (https://imechanica.org/node/24764),
August 2020: Mechanics of High-capacity Rechargeable Batteries (https://imechanica.org/node/24478),
November 2017: In-situ Mechanics Experiments on Battery Materials (https://imechanica.org/node/21788),
July 2016: Mechanics of Large-Volume-Change Materials for Rechargeable Batteries (https://imechanica.org/node/20073),
December 2015: Mechanics of Lithium Ion Battery Electrodes (https://imechanica.org/node/19189),
December 2010: Mechanics of Energy Storage (https://imechanica.org/node/9413).
So, what makes SSBs different? One quick answer is the solid/solid interfaces. In SSBs, the interfaces are the places where (contact) mechanics and electrochemistry meet each other and get coupled. This uniqueness has assigned a dominant role to mechanical characterization and engineering for SSBs. That is, in liquid-electrolyte batteries, mechanical deformation is more often modeled either as an unwanted effect responsible for battery degradation and failure or as a means to make special-purpose batteries such as flexible cells; but for SSBs, the cell performance and lifetime must be optimized through mechanical regulations. As a straightforward example, many research teams have reported that properly applying stack pressure on the surface of an SSB cell can significantly extend its lifetime [1]; but over-pressing the cell can directly cause an internal short circuit [2]. Therefore, an optimal level or range of stack pressure must be explored for SSB management.
There are two major types of interfaces co-existing in an SSB cell, i.e., the anode/solid electrolyte (SE) interface and the cathode/SE interface. It is worth noting that various material systems have been developed and reported for SSB applications. In other words, the materials used as anode, SE, and cathode vary from case to case. One general trend is clear - materials of high energy density are preferable for SSBs. Here, we use Li-metal-anode all-solid-state-battery (Li-ASSB) as an example. Figure 1 illusrtratively summarizes various failure mechanisms in a Li-ASSB cell. From our mechanical point of view, the fundmental challenges at the Li/SE and cathode/SE interfaces are different. At the Li/SE interface, the potential mechanisms of a chemo-mechanical failure can usually be identified, i.e., contact loss and lithium penetration. However, it is difficult to characterize the large deformation of pure Li through the grain boundaries (GBs) of SE. At the cathode/SE side, the challenge is that there are many potential failrue mechanisms. Cracks can happen between the polycrystalline microstructure of a single cathode particle and the SE (detachment), within the primary particles (intra-particle fracture), between primary particles (inter-particle fracture), or within the SE. In this journal club, we focus on the Li/SE side, referring the readers who are intereted in the the cathode/SE interface to some relevant papers [3-9].
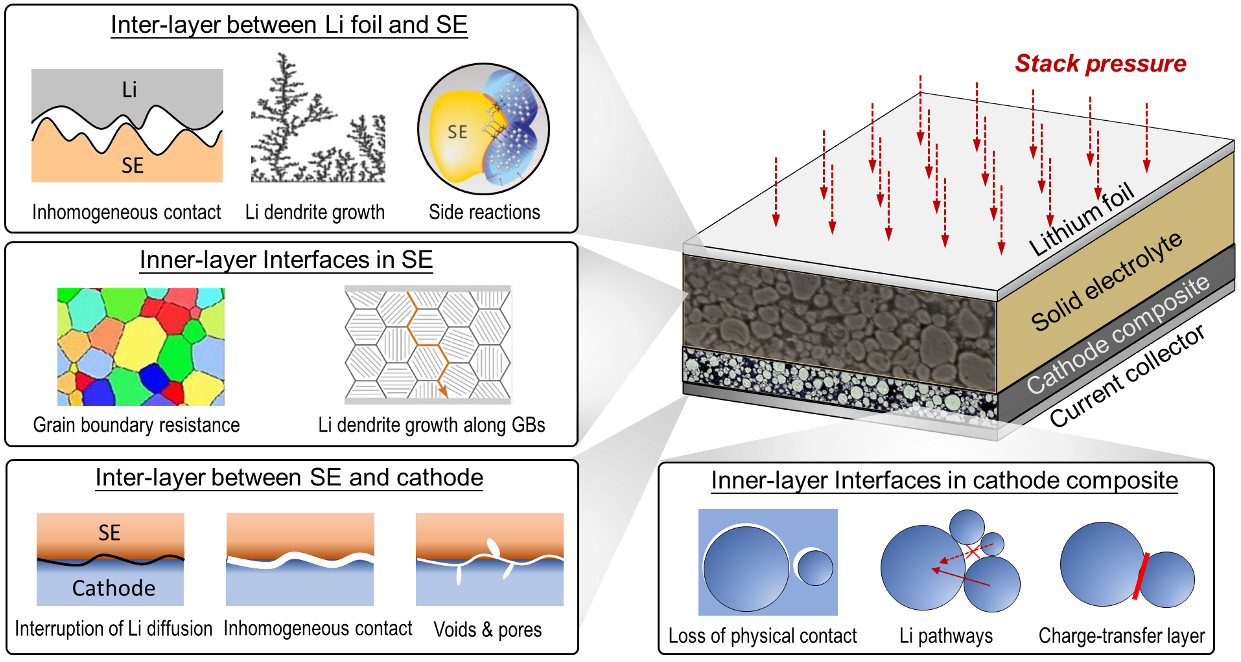
Figure 1. Various interfacial failure mechanisms in a Li-metal solid-state battery
2. Mechanical Properties of Component Materials2.1 Mechanical properties of solid electrolytes
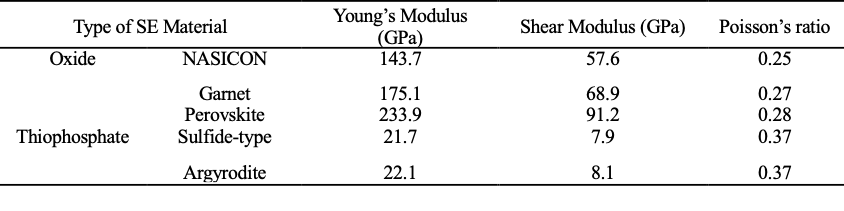
Developing advanced SEs is one of the key tasks for the SSB community. There were interesting debates in the community about whose choice is right and why competitors are wrong (e.g., The Problem with Sulfides | QuantumScape). So far, this is still an open question, and the two material systems currently being extensively studied are oxides (ceramics) and thiophosphates (sulfides). From a mechanical point of view, various materials lead to distinct mechanical behavior. Figure 2a plots the shear modulus vs. buld modulus of various SE materials [10]. Oxide solid electrolytes are typically stiff and brittle. The high Young’s modulus offers good resistance to mechanical load but with the drawback of easy perforation which can lead to short circuits. Applications of the three promising ceramics SE are NASICON, such as LiTi2(PO4)3, Garnet-type electrolytes, such as Li7La3Zr2O12 (LLZO), and Perovskite-type electrolytes, Li3xLa2/3-xTiO3. Figure 2b shows an exemplary SEM image of LLZO. Recently, another kind of SE, the thiophosphate solid electrolytes become more and more popular. Figure 2c shows an exemplary SEM image of Li6PS5Cl. Researchers expect this type of “softer” electrolyte to be able to accommodate large deformation while maintaining good electrode contact. We will elaborate on this comparison in Section 3.2 when talking about failure mechanisms.

Figure 2. (a) Pugh’s ratio of various SE materials [10], (b) SEM micrographs of LLZO ceramics powder [11], and (c) SEM cross-sections of the Li metal/Li6PS5Cl interface [12]
Table 1: Mechanical properties of different solid electrolyte materials. [10]
2.2 Mechanical deformation mechanisms of Li metal (as anode)
Compared with the SEs, Li metal is rather “softer” due to plastic flow, which leads to various interfacial failures [13]. In this section, we discuss how to characterize the mechanical behaviors of Li metal. As a monovalent alkali metal with a body-centered cubic structure, Li metal shows typical elastoplastic behaviors. Due to a low melting temperature of 453 K (180 C), Li metal has very complex mechanical behaviors concerning three major plastic deformation mechanisms: 1) the plastic flow by dislocation slip, 2) the power-law creep by dislocation climb, and 3) diffusional creep by vacancy diffusion through bulk and boundary. Each of the mechanisms dominates a range of mechanical conditions depending on the applied stress level and temperature. This can be described by a tool called “deformation-mechanism map” proposed by Frost and Ashby [14]. We reconstructed the deformation-mechanism map of Li Metal (Figure 3) following the method and parameters in [15] We can see three regions on the map where the contours of deformation rate are plotted against normalized shear stress and homologous temperature. The power-law creep dominates at lower applied stress levels, while slip-induced plasticity dominates at higher stress levels. A critical shear stress of around 1 MPa divides these two regions. The diffusional creep only contributes at much lower stress levels and the deformation rate is less significant. It is worth noting that Figure 3 is contructed using data reported in open literature [15] dated back in 1984. We look forward to seeing updated parameters with new experiemntal/theoretical results, as the material characterization and engineering technologies have improved significantly over the recent four decades.

Figure 3. The deformation mechanism map of lithium metal
Here, we would like to emphasize the permanent contributions of the community of solid mechanics to this important topic of understanding mechanical deformation mechanisms of pure Li. Pharr and co-workders have summarized the progress by 2020 as well as their own research [16] in a previous journal club (https://imechanica.org/node/24478). In addition, several experiment studies have reported on one or more of the deformation mechanisms in Figure 3. LePage et al. [13] conducted a set of uniaxial tension tests on bulk Li metal under different strain rates and temperatures (Figure 4a). They found that the deformation of Li metal follows the power-law creep at room temperature under a strain-rate between 4 × 10-5 to 3 × 10-3 s-1. Sedlatschek et al. [16] investigated the large deformation and fracture behaviors of Li metal through a set of miniature samples under a strain rate larger than 4 × 10-3 (Figure 4b). The rate-dependent strain-hardening is found to be the dominating factor in capturing the mechanical responses under various stress states.
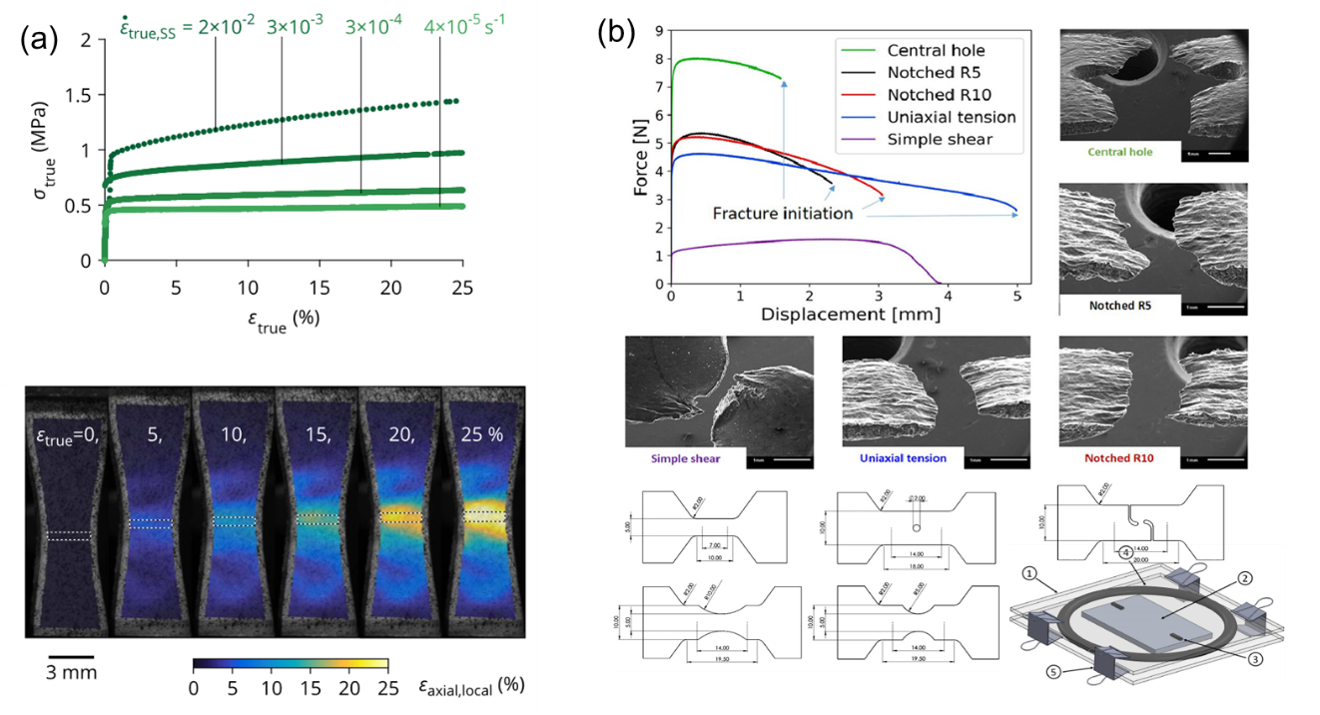
Figure 4. Mechanical tests on bulk Li metal. (a) Uniaxial tension tests showing the creep behavior at lower strain-rates [13]; (b) Large deformation and fracture tests under various stress states [17].
Another notable feature of Li Metal is the size-dependent mechanical behaviors. Figure 5 summarizes some representative experimental work on Li metal mechanical characterization at different length scales. It has been observed that the strength (yield stress) of Li metal increases as we decrease the size of the sample (Figure 5e). At the bulk scale, the yield stress is around 1 MPa, and it increases to 10 – 100 MPa at the micro scale.
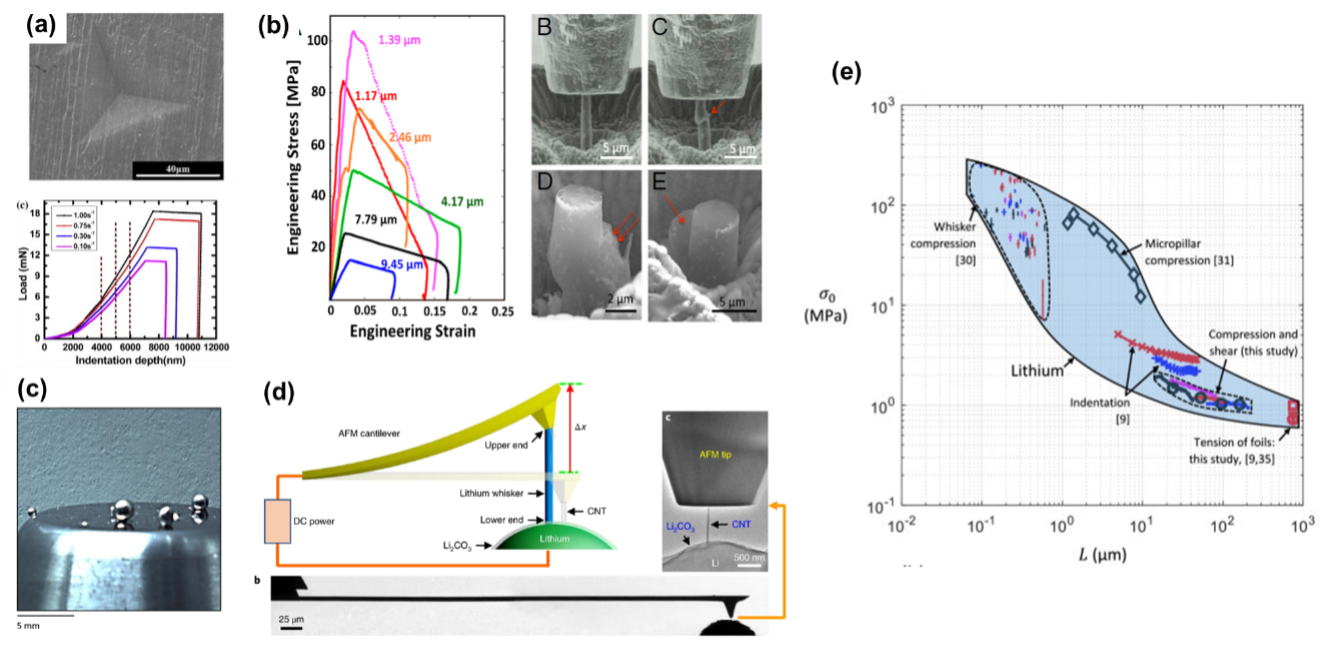
Figure 5. Size-dependent mechanical behaviors of Li metal. (a) Nanoindentation [18], (b) Micropolar compression [19], (c) Compression and shear of microspheres [20], (d) Whisker compression [21], (e) Yield strength of Li metal at different length scales [20].
Based on these experimental results, advanced mechanical models have been developed [22-24] and used in many studies (which will be mentioend in the following sections).
3. Interfacial failure
Interfacial contact loss and Li filament penetration are the two main interfacial failure phenomena, as illustrated in Figure 7. As the Li metal is removed, voids can form and grow, eventually leading to an interfacial contact loss [25]. This will cause a significant increase in the impedance of the batteries. On the other side, the Li filament would penetrate into the SE through the grain boundaries [26] and the imperfections at the SE surface [27]. With the accumulation of plated Li metal, cracks will grow and propagate within the SE. A newly formed crack serves as the new path for further Li penetration, eventually leading to an internal short circuit.

Figure 6. The SEM images of interfacial contact loss and Li penetration [28]
3.1 Void growth and loss of contact
The interfacial contact loss at the Li/SE interface can be attributed to the void formation and growth, which is governed by the competition between the vacancy diffusion within the Li [25,29–31] and the inelastic deformation of the Li [32,33]. During Li stripping, the Li/SE interface experiences the accumulation of vacancies due to diffusion. Excessive current density can lead to void formation if the vacancy concentration near the Li/SE interface surpasses the saturation threshold [25,34]. At elevated stack pressures externally applied on the battery surface, Li metal undergoes large plastic deformation due to its low yield strength [13,23,35]. The plastic ‘flow’ of Li metal could impede the void development, thus improving the interfacial stability.
3.2 Lithium penetration
Another source of interfacial failure in Li-ASSBs at the Li/SE interface is the penetration of Li into the SE, which can lead to an internal short circuit and catastrophic thermal runaway. Early rudimentary models, rooted in the elastic and no-flaw assumption of polymer separators [36], posited that increased stiffness could effectively mitigate lithium penetration. Inspired by this work, vast experimental studies on ceramic SEs have been reported [2,37]. However, the Li metal can still penetrate high-stiffness SEs through the grain boundaries (GBs) due to its higher electronic conductivity compared with SE grains [26]. Furthermore, due to the existence of initial imperfections such as pores and cracks in the SE, Li penetration can occur either during plating as a result of continuous reactions or when subjected to high stack pressure as a result of viscoplastic deformation [2,37]. The Li can gradually accumulate in the cracks or pores, leading to crack propagation and potentially an internal short circuit [27,38].
Li penetration depends on the electronic conductivity of the grain boundaries in the SE [26,39] and micro/nano-sized cracks at the SE surface [40–42], which is more likely to occur at high current density and pressure. The Li penetration into the cracks at the SE surface is identified as a more significant factor through some recent observations of the micro-scale Li plating experiment at the microprobe/SE interface using the microelectrode [40–42]. Based on the analytical expression derived from the structural and fracture mechanics, Klinsmann et al. [38] found that the pressure of inserted lithium metal into the cracks of the SE would accumulate to very high levels, leading to crack propagation due to the wedge-open mechanism until it reaches the cathode side. This process is well captured by the scanning X-ray computed tomography experiment by Ning et al.[27] It is worth mentioning that the Li metal could react with sulfide-based SE during operation, forming a buffering interface to impede the decomposition of SE. With continuous penetration, the inner part of SE could also decompose when they contact with the newly penetrated Li metal [43].
Here, we used the word "penetration" instead of the more widely known word "dendrites" because the latter is a result of the complex interplay of electrochemistry, mechanics, and material growth. We noticed a growing concern in the electrochemical community of this word being "abused". [19] and [21] set good examples by naming the test samples in Figure 5b and 5d as Li pillar and whisker, respectively. We use the word "penetration" to note that in many cases, lithium metal penetrate into SE driven by mechanical pressure, not necessarily a result of reactions and growth.
4. Multiphysics Modeling
The stripping and plating processes at the Li/SE interface are dominated by three physical processes—vacancy diffusion, interfacial electrochemical reactions, and plastic deformation of Li metal. Moreover, these processes are interconnected and often exhibit competing effects during the stripping and plating processes. In practice, maintaining the stable operation of Li-ASSB involves adjusting the applied stack pressure and current density post-manufacturing. Achieving an optimal range of pressure and current density for stable operation requires a theoretical model that considers the complex electro-chemo-mechanical interactions.
Two strategies are often used to model Li penetration and void evolution. One is the continuum-mechanical method that treats the boundaries as sharp interfaces [23,44–46] and the other is the phase-field method[31,39], which substitutes the sharp interfacial boundary by a partial differential equation for the evolution of the “phase field” described by “order parameters”. [23] proposed a continuum-mechanical model in which the Li plating and stripping at the Li/SE interface are simplified into the expansion and contraction of a thin-film layer between the Li and SE. The introduced thin-film layer could effectively describe the plating and stripping of Li metal through the continuum-mechanical method without directly modeling the transport of mass, as the reference configuration changes. Agier et al. [47] explored the void evolution process based on a continuum-mechanical model and emphasized that the imperfection particles within the Li are the main cause of the void growth and initiation. Shishvan et al.[45] proposed a continuum-mechanical model to discuss the effect of void diffusion and lattice drift on the void evolution and found that the void will not grow due to the lattice drift, which is contradictory to some experimental observations[25,29]. Treating the penetrated Li filament as a climbing edge dislocation, Shishvan et al. [48] calculate the growth rate of the Li filament at different applied plating current densities using an analytical model, which could be used to predict the critical current density for the Li penetration. In summary, it is challenging to comprehensively describe the intricate interplay between electrochemical processes and mechanics at the Li/SE interface using the conventional continuum-mechanical method. Delegate treatments must be developed, such as considering plating/stripping as the expansion/contraction of an artificial thin film[23] or analogizing Li penetration to a climbing dislocation [49]. This makes it difficult to establish a general and unified model for the stripping and plating process at the Li/SE interface.
The phase-field method has been adopted in analyzing lithium plating phenomenon in conventional liquid Li-ion batteries [50–54]. Recently, the method has been extended to all-solid-state Li-ion batteries. Tian et al. [39] proposed a phase-field model to study the electrodeposition process of Li in the grain boundary of the SE. In their work, the different SE grains, Li metal, grain boundaries, and cracks are represented by a set of order parameters, and the computational model revealed that the isolated Li deposition within the GBs can be caused by its higher electronic conductivity. Zhao et al. [31] proposed a thermodynamically consistent phase-field model to study the void evolution during the stripping and plating process, considering the effect of vacancy accumulation at the Li/SE interface. The reaction current concentration at the Li/void/SE triple phase was identified as the cause of isolated void formation during continuous stripping/plating cycling [31]. These recent studies highlighted the capability of the phase-field method to effectively capture complex electro-chemo-mechanical interactions respectively for Li penetration and void evolution, shedding light on characterizing the two dominant failure mechanisms in a unified framework.
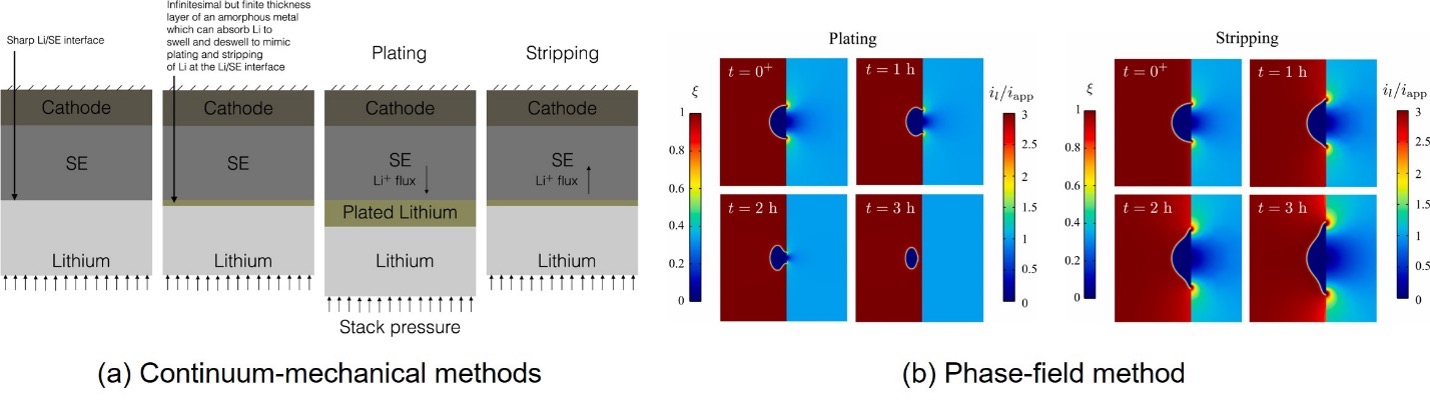
Figure 8. Representative work using the two modeling methods [23,31]. (a) is the work using the continuum-mechanical method to simulate the plating and stripping reaction at Li/SE interface. (b) used the phase-field method to simulate the void evolution during plating and stripping process
5. Concluding remarks
Mechanics plays a dominant role in the design, characterization, and manufacturing of solid-state batteries. In this short journal club article, we only managed to cover several typical examples related to the large deformation at the Li/SE interface. A very important topic that is neglected here is the fracture behavior of SEs. Intrinsically, it is challenging to experimentally measure fracture-related physical parameters [55], but recent publications from the mechanics community shed light on this important topic, for example, [40,41,56,57].
To conclude this article and open up the interactive discussions (comments below), let’s return to the Introduction section. It is clear that SSBs are a promising type of next-generation energy storage systems. If there were a third fold of implication behind the word “next-generation,” we believe it must be – Whether this new technology can be successfully developed and commercialized largely depends on the efforts of researchers in the CURRENT GENERATION. We are excited to see the community of mechanics leading in so many important challenges.
** This journal club article is prepared by extending the introduction section of our manuscript in preparation. As of 1/31/2024, the manuscript is undergoing our final round of internal improvement before formal submission. We were convinced by the iMechanica Journal Club Editor who told us “iMechanica Journal Club has more professional readers than a preprint.” We will add the DOI record of the manuscript once it gets published.
References
[1] J.-M. Doux, Y. Yang, D.H.S. Tan, H. Nguyen, E.A. Wu, X. Wang, A. Banerjee, Y.S. Meng, Pressure effects on sulfide electrolytes for all solid-state batteries, J. Mater. Chem. A 8 (2020) 5049–5055. https://doi.org/10.1039/C9TA12889A.
[2] J.M. Doux, H. Nguyen, D.H.S. Tan, A. Banerjee, X.F. Wang, E.A. Wu, C. Jo, H.D. Yang, Y.S. Meng, Stack Pressure Considerations for Room-Temperature All-Solid-State Lithium Metal Batteries, Adv. Energy Mater. 10 (2020). https://doi.org/10.1002/aenm.201903253.
[3] R. Xu, Y. Yang, F. Yin, P. Liu, P. Cloetens, Y. Liu, F. Lin, K. Zhao, Heterogeneous damage in Li-ion batteries: Experimental analysis and theoretical modeling, J. Mech. Phys. Solids 129 (2019) 160–183. https://doi.org/10.1016/j.jmps.2019.05.003.
[4] L.S. de Vasconcelos, R. Xu, Z. Xu, J. Zhang, N. Sharma, S.R. Shah, J. Han, X. He, X. Wu, H. Sun, S. Hu, M. Perrin, X. Wang, Y. Liu, F. Lin, Y. Cui, K. Zhao, Chemomechanics of Rechargeable Batteries: Status, Theories, and Perspectives, Chem. Rev. 122 (2022) 13043–13107. https://doi.org/10.1021/acs.chemrev.2c00002.
[5] Y. Mao, X. Wang, S. Xia, K. Zhang, C. Wei, S. Bak, Z. Shadike, X. Liu, Y. Yang, R. Xu, P. Pianetta, S. Ermon, E. Stavitski, K. Zhao, Z. Xu, F. Lin, X. Yang, E. Hu, Y. Liu, High‐Voltage Charging‐Induced Strain, Heterogeneity, and Micro‐Cracks in Secondary Particles of a Nickel‐Rich Layered Cathode Material, Adv. Funct. Mater. 29 (2019) 1900247. https://doi.org/10.1002/adfm.201900247.
[6] A. Singh, S. Pal, Coupled chemo-mechanical modeling of fracture in polycrystalline cathode for lithium-ion battery, Int. J. Plast. 127 (2020) 102636. https://doi.org/10.1016/j.ijplas.2019.11.015.
[7] J. Han, N. Sharma, K. Zhao, Computational modeling of coupled mechanical damage and electrochemistry in ternary oxide composite electrodes, J. Power Sources 595 (2024) 234034. https://doi.org/10.1016/j.jpowsour.2023.234034.
[8] A. Singh, S. Pal, Chemo-mechanical modeling of inter- and intra-granular fracture in heterogeneous cathode with polycrystalline particles for lithium-ion battery, J. Mech. Phys. Solids 163 (2022) 104839. https://doi.org/10.1016/j.jmps.2022.104839.
[9] A. Singh, J. Song, W. Li, T. Martin, H. Xu, D. Finegan, J. Zhu, Microstructure-Chemomechanics Relations of Polycrystalline Cathodes in Solid-State Batteries, SSRN, 2024. https://doi.org/10.2139/ssrn.4707191.
[10] Z. Deng, Z. Wang, I.-H. Chu, J. Luo, S.P. Ong, Elastic Properties of Alkali Superionic Conductor Electrolytes from First Principles Calculations, J. Electrochem. Soc. 163 (2015) A67. https://doi.org/10.1149/2.0061602jes.
[11] M. Botros, R. Djenadic, O. Clemens, M. Möller, H. Hahn, Field assisted sintering of fine-grained Li7−3xLa3Zr2AlxO12 solid electrolyte and the influence of the microstructure on the electrochemical performance, J. Power Sources 309 (2016) 108–115. https://doi.org/10.1016/j.jpowsour.2016.01.086.
[12] J. Kasemchainan, S. Zekoll, D. Spencer Jolly, Z. Ning, G.O. Hartley, J. Marrow, P.G. Bruce, Critical stripping current leads to dendrite formation on plating in lithium anode solid electrolyte cells, Nat. Mater. 18 (2019) 1105–1111. https://doi.org/10.1038/s41563-019-0438-9.
[13] W.S. LePage, Y.X. Chen, E. Kazyak, K.H. Chen, A.J. Sanchez, A. Poli, E.M. Arruda, M.D. Thouless, N.P. Dasgupta, Lithium Mechanics: Roles of Strain Rate and Temperature and Implications for Lithium Metal Batteries, J. Electrochem. Soc. 166 (2019) A89–A97. https://doi.org/10.1149/2.0221902jes.
[14] Harold H. Frost, Michael F. Ashby, Deformation-mechanism Maps: The Plasticity and Creep of Metals and Ceramics, PERGAMON PRESS, 1980.
[15] P.M. Sargent, M.F. Ashby, Deformation mechanism maps for alkali metals, Scr. Metall. 18 (1984) 145–150. https://doi.org/10.1016/0036-9748(84)90494-0.
[16] C.D. Fincher, D. Ojeda, Y. Zhang, G.M. Pharr, M. Pharr, Mechanical properties of metallic lithium: from nano to bulk scales, Acta Mater. 186 (2020) 215–222. https://doi.org/10.1016/j.actamat.2019.12.036.
[17] T. Sedlatschek, J. Lian, W. Li, M. Jiang, T. Wierzbicki, M.Z. Bazant, J. Zhu, Large-deformation plasticity and fracture behavior of pure lithium under various stress states, Acta Mater. 208 (2021) 116730. https://doi.org/10.1016/j.actamat.2021.116730.
[18] Y. Wang, Y.-T. Cheng, A nanoindentation study of the viscoplastic behavior of pure lithium, Scr. Mater. 130 (2017) 191–195. https://doi.org/10.1016/j.scriptamat.2016.12.006.
[19] C. Xu, Z. Ahmad, A. Aryanfar, V. Viswanathan, J.R. Greer, Enhanced strength and temperature dependence of mechanical properties of Li at small scales and its implications for Li metal anodes, Proc. Natl. Acad. Sci. 114 (2017) 57–61. https://doi.org/10.1073/pnas.1615733114.
[20] J.C. Stallard, S. Vema, C.P. Grey, V.S. Deshpande, N.A. Fleck, The strength of a constrained lithium layer, Acta Mater. 260 (2023) 119313. https://doi.org/10.1016/j.actamat.2023.119313.
[21] L. Zhang, T. Yang, C. Du, Q. Liu, Y. Tang, J. Zhao, B. Wang, T. Chen, Y. Sun, P. Jia, H. Li, L. Geng, J. Chen, H. Ye, Z. Wang, Y. Li, H. Sun, X. Li, Q. Dai, Y. Tang, Q. Peng, T. Shen, S. Zhang, T. Zhu, J. Huang, Lithium whisker growth and stress generation in an in situ atomic force microscope–environmental transmission electron microscope set-up, Nat. Nanotechnol. 15 (2020) 94–98. https://doi.org/10.1038/s41565-019-0604-x.
[22] S. Narayan, L. Anand, A large deformation elastic–viscoplastic model for lithium, Extreme Mech. Lett. 24 (2018) 21–29. https://doi.org/10.1016/j.eml.2018.08.006.
[23] S. Narayan, L. Anand, On Modeling the Detrimental Effects of Inhomogeneous Plating-and-Stripping at a Lithium-Metal/Solid-Electrolyte Interface in a Solid-State-Battery, J. Electrochem. Soc. 167 (2020). https://doi.org/10.1149/1945-7111/ab75c1.
[24] L. Anand, S. Narayan, An Elastic-Viscoplastic Model for Lithium, J. Electrochem. Soc. 166 (2019) A1092–A1095. https://doi.org/10.1149/2.0861906jes.
[25] T. Krauskopf, H. Hartmann, W.G. Zeier, J. Janek, Toward a Fundamental Understanding of the Lithium Metal Anode in Solid-State Batteries-An Electrochemo-Mechanical Study on the Garnet-Type Solid Electrolyte Li(6.25)Al(0.25)La(3)Zr(2)O(12), ACS Appl Mater Interfaces 11 (2019) 14463–14477. https://doi.org/10.1021/acsami.9b02537.
[26] F.D. Han, A.S. Westover, J. Yue, X.L. Fan, F. Wang, M.F. Chi, D.N. Leonard, N. Dudney, H. Wang, C.S. Wang, High electronic conductivity as the origin of lithium dendrite formation within solid electrolytes, Nat. Energy 4 (2019) 187–196. https://doi.org/10.1038/s41560-018-0312-z.
[27] Z. Ning, G. Li, D.L.R. Melvin, Y. Chen, J. Bu, D. Spencer-Jolly, J. Liu, B. Hu, X. Gao, J. Perera, C. Gong, S.D. Pu, S. Zhang, B. Liu, G.O. Hartley, A.J. Bodey, R.I. Todd, P.S. Grant, D.E.J. Armstrong, T.J. Marrow, C.W. Monroe, P.G. Bruce, Dendrite initiation and propagation in lithium metal solid-state batteries, Nature 618 (2023) 287–293. https://doi.org/10.1038/s41586-023-05970-4.
[28] L. Zhao, W. Li, C. Wu, Q. Ai, L. Guo, Z. Chen, J. Zheng, M. Anderson, H. Guo, J. Lou, Y. Liang, Z. Fan, J. Zhu, Y. Yao, Taming Metal–Solid Electrolyte Interface Instability via Metal Strain Hardening, Adv. Energy Mater. (2023). https://doi.org/10.1002/aenm.202300679.
[29] T. Krauskopf, B. Mogwitz, C. Rosenbach, W.G. Zeier, J. Janek, Diffusion Limitation of Lithium Metal and Li–Mg Alloy Anodes on LLZO Type Solid Electrolytes as a Function of Temperature and Pressure, Adv. Energy Mater. 9 (2019). https://doi.org/10.1002/aenm.201902568.
[30] C.-T. Yang, Y. Qi, Maintaining a Flat Li Surface during the Li Stripping Process via Interface Design, Chem. Mater. 33 (2021) 2814–2823. https://doi.org/10.1021/acs.chemmater.0c04814.
[31] Y. Zhao, R. Wang, E. Martínez-Pañeda, A phase field electro-chemo-mechanical formulation for predicting void evolution at the Li–electrolyte interface in all-solid-state batteries, J. Mech. Phys. Solids 167 (2022). https://doi.org/10.1016/j.jmps.2022.104999.
[32] M. Feng, C.-T. Yang, Y. Qi, The Critical Stack Pressure to Alter Void Generation at Li/Solid-Electrolyte Interfaces during Stripping, J. Electrochem. Soc. 169 (2022). https://doi.org/10.1149/1945-7111/ac91aa.
[33] Y. Lu, C.Z. Zhao, J.K. Hu, S. Sun, H. Yuan, Z.H. Fu, X. Chen, J.Q. Huang, M.G. Ouyang, Q. Zhang, The void formation behaviors in working solid-state Li metal batteries, Sci Adv 8 (2022). https://doi.org/ARTN eadd0510 10.1126/sciadv.add0510.
[34] H. Schmalzried, J. Janek, Chemical kinetics of phase boundaries in solids, Berichte Bunsen-Ges.-Phys. Chem. Chem. Phys. 102 (1998) 127–143. https://doi.org/DOI 10.1002/bbpc.19981020202.
[35] T. Sedlatschek, J. Lian, W. Li, M. Jiang, T. Wierzbicki, M.Z. Bazant, J. Zhu, Large-deformation plasticity and fracture behavior of pure lithium under various stress states, Acta Mater. 208 (2021). https://doi.org/10.1016/j.actamat.2021.116730.
[36] C. Monroe, J. Newman, The Impact of Elastic Deformation on Deposition Kinetics at Lithium/Polymer Interfaces, J. Electrochem. Soc. 152 (2005) A396. https://doi.org/10.1149/1.1850854.
[37] D. Cao, K. Zhang, W. Li, Y. Zhang, T. Ji, X. Zhao, E. Cakmak, J. Zhu, Y. Cao, H. Zhu, Nondestructively Visualizing and Understanding the Mechano‐Electro‐chemical Origins of “Soft Short” and “Creeping” in All‐Solid‐State Batteries, Adv. Funct. Mater. (2023). https://doi.org/10.1002/adfm.202307998.
[38] M. Klinsmann, F.E. Hildebrand, M. Ganser, R.M. McMeeking, Dendritic cracking in solid electrolytes driven by lithium insertion, J. Power Sources 442 (2019). https://doi.org/10.1016/j.jpowsour.2019.227226.
[39] H.-K. Tian, Z. Liu, Y. Ji, L.-Q. Chen, Y. Qi, Interfacial Electronic Properties Dictate Li Dendrite Growth in Solid Electrolytes, Chem. Mater. 31 (2019) 7351–7359. https://doi.org/10.1021/acs.chemmater.9b01967.
[40] C.E. Athanasiou, C.D. Fincher, C. Gilgenbach, H. Gao, W.C. Carter, Y.-M. Chiang, B.W. Sheldon, Operando measurements of dendrite-induced stresses in ceramic electrolytes using photoelasticity, Matter 7 (2024) 95–106. https://doi.org/10.1016/j.matt.2023.10.014.
[41] C.D. Fincher, C.E. Athanasiou, C. Gilgenbach, M. Wang, B.W. Sheldon, W.C. Carter, Y.-M. Chiang, Controlling dendrite propagation in solid-state batteries with engineered stress, Joule 6 (2022) 2794–2809. https://doi.org/10.1016/j.joule.2022.10.011.
[42] G. McConohy, X. Xu, T. Cui, E. Barks, S. Wang, E. Kaeli, C. Melamed, X.W. Gu, W.C. Chueh, Mechanical regulation of lithium intrusion probability in garnet solid electrolytes, Nat. Energy (2023). https://doi.org/10.1038/s41560-022-01186-4.
[43] S. Luo, Z. Wang, X. Li, X. Liu, H. Wang, W. Ma, L. Zhang, L. Zhu, X. Zhang, Growth of lithium-indium dendrites in all-solid-state lithium-based batteries with sulfide electrolytes, Nat. Commun. 12 (2021) 6968. https://doi.org/10.1038/s41467-021-27311-7.
[44] S.S. Shishvan, N.A. Fleck, V.S. Deshpande, The initiation of void growth during stripping of Li electrodes in solid electrolyte cells, J. Power Sources 488 (2021). https://doi.org/10.1016/j.jpowsour.2020.229437.
[45] S.S. Shishvan, N.A. Fleck, R.M. McMeeking, V.S. Deshpande, Vacancy diffusion and its consequences for void growth at the interface of a stripping metal electrode and solid electrolyte, Electrochimica Acta 467 (2023). https://doi.org/10.1016/j.electacta.2023.143081.
[46] S.S. Shishvan, N.A. Fleck, R.M. McMeeking, V.S. Deshpande, Void growth in metal anodes in solid-state batteries: Recent progress and gaps in understanding, Eur. J. Mech. - ASolids 100 (2023). https://doi.org/10.1016/j.euromechsol.2023.104998.
[47] J.A.B. Agier, S.S. Shishvan, N.A. Fleck, V.S. Deshpande, Void growth within Li electrodes in solid electrolyte cells, Acta Mater. 240 (2022). https://doi.org/10.1016/j.actamat.2022.118303.
[48] S.S. Shishvan, N.A. Fleck, R.M. McMeeking, V.S. Deshpande, Growth rate of lithium filaments in ceramic electrolytes, Acta Mater. 196 (2020) 444–455. https://doi.org/10.1016/j.actamat.2020.06.060.
[49] S.S. Shishvan, N.A. Fleck, R.M. McMeeking, V.S. Deshpande, Dendrites as climbing dislocations in ceramic electrolytes: Initiation of growth, J. Power Sources 456 (2020). https://doi.org/ARTN 227989 10.1016/j.jpowsour.2020.227989.
[50] L. Chen, H.W. Zhang, L.Y. Liang, Z. Liu, Y. Qi, P. Lu, J. Chen, L.-Q. Chen, Modulation of dendritic patterns during electrodeposition: A nonlinear phase-field model, J. Power Sources 300 (2015) 376–385. https://doi.org/10.1016/j.jpowsour.2015.09.055.
[51] L. Gao, Z. Guo, Phase-field simulation of Li dendrites with multiple parameters influence, Comput. Mater. Sci. 183 (2020). https://doi.org/10.1016/j.commatsci.2020.109919.
[52] Z. Hong, V. Viswanathan, Phase-Field Simulations of Lithium Dendrite Growth with Open-Source Software, ACS Energy Lett. 3 (2018) 1737–1743. https://doi.org/10.1021/acsenergylett.8b01009.
[53] J. Zhang, Y. Liu, C. Wang, H. Tan, An Electrochemical-Mechanical Phase Field Model for Lithium Dendrite, J. Electrochem. Soc. 168 (2021). https://doi.org/10.1149/1945-7111/ac22c7.
[54] R. Zhang, X. Shen, Y.-T. Zhang, X.-L. Zhong, H.-T. Ju, T.-X. Huang, X. Chen, J.-D. Zhang, J.-Q. Huang, Dead lithium formation in lithium metal batteries: A phase field model, J. Energy Chem. 71 (2022) 29–35. https://doi.org/10.1016/j.jechem.2021.12.020.
[55] J. Wolfenstine, H. Jo, Y.-H. Cho, I.N. David, P. Askeland, E.D. Case, H. Kim, H. Choe, J. Sakamoto, A preliminary investigation of fracture toughness of Li7La3Zr2O12 and its comparison to other solid Li-ionconductors, Mater. Lett. 96 (2013) 117–120. https://doi.org/10.1016/j.matlet.2013.01.021.
[56] C.E. Athanasiou, X. Liu, M.Y. Jin, E. Nimon, S. Visco, C. Lee, M. Park, J. Yun, N.P. Padture, H. Gao, B.W. Sheldon, Rate-dependent deformation of amorphous sulfide glass electrolytes for solid-state batteries, Cell Rep. Phys. Sci. 3 (2022) 100845. https://doi.org/10.1016/j.xcrp.2022.100845.
[57] S. Monismith, C.D. Fincher, Y. Chiang, J. Qu, R. Dingreville, Harnessing Electrochemical‐Mechanical Couplings to Improve the Reliability of Solid‐State Batteries, Adv. Energy Mater. (2023) 2303567. https://doi.org/10.1002/aenm.202303567.
- Juner Zhu's blog
- Log in or register to post comments
- 3887 reads


Comments
Li penetration through solid electrolytes
Dear Juner,
Thank you for leading the journal club discussion on the mechanics of solid-state batteries. The review your team provided is timely and insightful. I am very interested in the issue of Li penetration through solid electrolytes. You mentioned the possibility of Li penetration through grain boundaries of solid electrolytes. Is there direct experimental evidence of such penetration?
Best regards,
Ting
Re: Li penetration through solid electrolytes
Dear Ting,
Thank you for your kind words. Yes, Li penetration through SE is a very interesting mechanics problem. Direct experimental observations are actually limited due to the difficulty in testing, in situ, operando, or post-mortem. To our knowledge, the 2017 Electrochem. Acta paper by Jeff Sakamoto's team provided such an example:
The electrolyte tested in their study is LLZO.
Yet-Ming Chiang's group also has many direct observations: https://doi.org/10.1002/aenm.201701003.
And also these ones for direct and in-direct observations.
https://doi.org/10.1038/s41598-020-75456-0
https://doi.org/10.1002/aenm.202000702
https://doi.org/10.1002/adfm.202307998
Young's Modulus vs.Tensile Strength Chart of Several Materials
Dear Juner ZHU,
What are the References of the Young's Modulus vs.Tensile Strength E(σY) Chart of Metals, Alloys, Polymers, Elastomers, Ceramics and Glasses available on Google ?
Mohammed Lamine MOUSSAOUI
Hi Mohammed,
Hi Mohammed,
I wonder which figure you are referring to. Could you specify the number?
Best,
Juner
Figure
Hi Juner,
There are several figures and espacially that is available with the following link
https://www.google.dz/url?sa=i&url=https%3A%2F%2Fwww.ansys.com%2Fit-it%2Facademic%2Feducators%2Feducation-resources%2Fchart-youngs-modulus-vs-tensile-strength&psig=AOvVaw1-R47NBexD55BjykkmzVDc&ust=1709257511119000&source=images&cd=vfe&opi=89978449&ved=0CBIQjRxqFwoTCKiI7pW3z4QDFQAAAAAdAAAAABAJ
We want to know its origins
Regards
Mohammed lamine