You are here
Journal Club for April 2024: The mechanics and mechanisms of hydrogen embrittlement
The mechanics and mechanisms of hydrogen embrittlement
Emilio Martinez-Paneda. University of Oxford
1. Introduction
When first observed, the interaction between hydrogen and metals was described as “remarkable” and “extraordinary” [1]. When metallic materials are exposed to a hydrogen-containing environment, hydrogen is absorbed into the material and this dissolved hydrogen causes a dramatic degradation in mechanical properties, a phenomenon referred to as hydrogen embrittlement [2]. As little as a few parts per million (ppm) of hydrogen can change – by orders of magnitude – a metal’s ductility (elongation), fracture toughness, and fatigue crack growth rates [3]. This is exemplified in Fig. 1. Namely, Fig. 1a shows how the ductility of a pipeline steel (ASME SA106) is significantly reduced when exposed to a hydrogen-containing environment. The exposure of ASME SA106 samples to a gaseous hydrogen environment with an H2 pressure of 24.2 MPa halves their failure strain, relative to the hydrogen-free condition. Fig. 1b shows how the fracture toughness of a pressure vessel steel (21/4Cr-1Mo steel) decreases dramatically with increasing hydrogen content, from a hydrogen-free value above 200 MPa√m to values below 20 MPa√m for hydrogen contents exceeding 6 ppm [4, 5]. Fig. 1c shows how fatigue cracks grow orders of magnitude faster in pipeline steels when they are exposed to hydrogen gas (21 MPa H2 pressure), relative to the results attained in air [6]. These macroscopic observations have been consistently and widely reported for a very wide range of metals and alloys.

Figure 1: Exemplifying the dramatic effect of hydrogen: (a) ductility reduction of a pipeline steel (ASME SA106) when exposed to hydrogen gas environments [7, 8]; (b) fracture toughness reduction of a 21/4Cr-1Mo steel versus hydrogen content, showing a significant drop from a hydrogen-free value that is typically above 200 MPa√m (see, e.g. [5]); and (c) fatigue crack growth rates (da/dN) for various pipeline steels as a function of the stress intensity factor range ∆K in the absence (air) and presence of hydrogen gas (21 MPa H2)[6] – crack growth rates increase by more than one order of magnitude when samples are exposed to hydrogen. Data from Refs. [6, 7].
The phenomenon of hydrogen embrittlement has attracted the attention of the material science and solid mechanics communities for decades due to its scientific complexity and its important technological implications - hydrogen-assisted failures are pervasive across the transport, defence, energy, and construction sectors [9, 10]. Moreover, the problem has come very much to the fore in recent years as a consequence of the higher susceptibility of new, high-strength alloys, and because of the promise that hydrogen holds as a future energy carrier, requiring the development of suitable structures for hydrogen storage and transport [3, 11]. Hydrogen embrittlement is considered one of the biggest impediments to the broader implementation of a hydrogen-based fuel economy, hindering the transition away from fossil fuels. However, 150 years after this phenomenon was first observed [1], its underlying physical mechanisms are as debated as ever, hindering the prediction of hydrogen-assisted failures and the development of hydrogen-resistant materials. It should be emphasised that numerous excellent contributions have been made in this field over the past 150 years and most will be omitted here, as the goal of this iMechanica Journal Club contribution is not to review the state-of-the-art but to provide a brief, critical overview of hydrogen embrittlement research and introduce the topic to the wider community of mechanicians since, common to many other important problems, mechanics sits at its core.
2. Understanding and predicting hydrogen embrittlement
Hydrogen embrittlement is notorious for its complexity, as it spans multiple scales and disciplines (mechanics, materials science, chemistry). Fig. 2 illustrates the key stages and physical processes involved in hydrogen embrittlement. Firstly, hydrogen is absorbed into the metal from the environment. Secondly, the atomic hydrogen dissolved in the metal either diffuses freely via interstitial lattice sites or is trapped in pre-existing defects like grain boundaries, carbides and dislocations. The former diffuses towards regions of high hydrostatic stress, such as crack tips. And finally, the interaction of hydrogen and mechanical fields in the fracture process zone facilitates the nucleation and growth of cracks, through mechanisms that are still a subject of intense research. These steps are described below, emphasising the key role that mechanics plays in each of them.

Figure 2: Hydrogen uptake, transport and embrittlement. First, hydrogen adsorption and absorption take place following molecular dissociation (gas) or proton/water reduction (aqueous electrolyte). Then, atomic hydrogen diffuses freely through the crystal lattice or it is sequestered at microstructural traps such as grain boundaries, dislocations and carbides. The lattice hydrogen is attracted to areas of high hydrostatic stress such as crack tips, where the fracture energy of the material is reduced through multi-scale embrittlement mechanisms.
2.1 Hydrogen ingress
Hydrogen ingress into a metal can happen during its initial forming, during the coating or plating of a protective layer, through exposure to hydrogen or hydrogen-containing molecules in the air, soil or water, or through corrosion processes. For the sake of brevity, the focus is here on hydrogen uptake from gaseous environments, as other scenarios are more complex and require a comprehensive electro-chemo-mechanical treatment. The reader is referred to Refs. [12–14] for a rigorous description of more general conditions, including hydrogen uptake from aqueous electrolytes.
Under steady state conditions, the absorption of hydrogen into a metal can be quantified using Sievert’s law, which relates the lattice hydrogen concentration (CL) with the material solubility S and the fugacity of the H2 environment (fH2), as

The fugacity is a measure of the severity of the environment and is equal to the H2 pressure (pH2) for low pressures but deviates for higher ones, where the Able-Noble equation can be used to relate pH2 and fH2 [15]. The solubility, on the other hand, is a material parameter that is typically determined from experimental measurements of diffusivity and permeability. Importantly, the solubility depends on the stress state at the subsurface of the material.
As described below (Section 2.2), hydrostatic stresses (or volumetric strains) expand the crystal lattice, facilitating the diffusion and absorption of hydrogen atoms. Considering equilibrium conditions, the chemical potential of the environment (µH2) must be equal to the chemical potential of the hydrogen in the lattice (µL). And since the H2 molecule contains two hydrogen atoms (1/2H2 ⇔ H), one reaches,

The definition of the chemical potential for the H2 environment and the lattice are respectively given by (see, e.g., [16]):

where the variables with superscript 0 denote reference quantities (µ0H2, µ0L, p0), and the term VHσH accounts for the role that deformation plays in the transport of hydrogen, with σH being the hydrostatic stress and VH being the partial molar volume of hydrogen in solid solution. Since hydrogen diffuses from regions of high chemical potential to those with low chemical potential, the term VHσH results in an accumulation of hydrogen in regions of high hydrostatic stress. In addition, the term θL is the occupancy of hydrogen in the lattice, which is related to the density of lattice sites and the hydrogen concentration in the lattice as θL = CL/NL. Considering that the chemical potential of hydrogen gas in the reference or standard state (p0 = 1 atm, T = 25◦C) is zero, one can substitute (3) and (4) into (2), and obtain

The coupling with mechanics becomes evident, with the absorbed hydrogen content being scaled by an exponential term growing with the hydrostatic stress. This is of critical importance as solubility measurements are typically conducted in unstressed samples and therefore can significantly underestimate the uptake of hydrogen ahead of cracks and other stress concentrators.
2.2 Hydrogen transport
We deal with the transport of diluted species; that is, the concentration of hydrogen is small compared to the concentration of the metal solvent. Hydrogen atoms occupy normal interstitial lattice sites and additionally can reside at trapping sites such as interfaces or dislocations. The subscript L refers to lattice sites and the subscript T to trap sites. It is commonly assumed that traps are isolated (i.e., do not form an extended network). Hence, hydrogen transport between trap sites is by lattice diffusion. Traps act as hydrogen sinks, slowing diffusion, and are typically characterised by their binding energy WB and density NT. The energy barrier that must be overcome for the hydrogen to detrap increases with |WB|; hydrogen will be strongly retained in deep traps |WB| > 60 kJ/mol but can be easily released from shallow traps |WB| < 30 kJ/mol. Quantifying this partitioning of hydrogen atoms between lattice and trapping sites is of utmost importance in predicting diffusion and embrittlement; see, e.g., [17–19] and references therein.
We have already defined the concentration of hydrogen atoms in the lattice,

as a function of the occupancy (θL) and the density of lattice sites (NL). The latter is a function of the molar volume of the host lattice (VM), the number of interstitial sites per lattice site (β) and Avogadro’s number (NA), such that NL = βNA/VM. For bcc metals, β = 6 and NL = 5.1×1029 sites/m3. On the other hand, the concentration of hydrogen trapped in microstructural defects is denoted as C(i)T, where (i) refers to the trap type (e.g., dislocations, grain boundaries, interfaces), such that,
![]()
where NT is the trap density and θT the trap occupancy. The trap density is an intrinsic material characteristic that remains constant for traps such as carbides or grain boundaries but that evolves with mechanical load and plastic straining for the case of dislocation traps, establishing another important coupling with mechanics. The total hydrogen content is thus given by C = CL + Σi C(i)T.
It is common to assume that a thermodynamic equilibrium exists between the lattice and trapped hydrogen. This equilibrium, first formulated by Oriani [20], assumes the following Fermi-Dirac relationship between the trap and lattice occupancies,
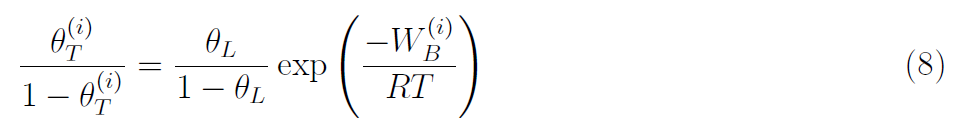
where W(i)B is the binding energy for a trap of type (i). The implications of Oriani’s equilibrium can be observed in Fig. 3, where Eqs. (6)-(8) have been combined to plot contours of trap occupancy θT as a function of the binding energy and the lattice hydrogen content [19, 21]. Traps with binding energies below -50 kJ/mol saturate at very low values of CL, while traps with binding energies higher than -20 kJ/mol are essentially empty (θT ≈ 0) unless CL is very high, on the order of 10 wt ppm (4.68 × 1025 at H/m3) or higher.

Figure 3: Implications of Oriani’s equilibrium; sensitivity of the trap occupancy θT to the lattice hydrogen concentration CL and the trap binding energy WB. Taken from Ref. [19].
Traps hinder hydrogen diffusivity and this can be captured through the definition of an effective diffusion coefficient that considers the contribution from all trap types, such that
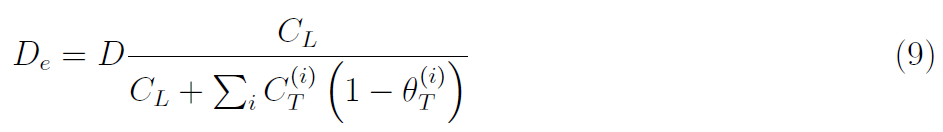
And consequently, the mass balance equation that describes the evolution of hydrogen in time and space is given by [22],

Other approaches also exist, including those based on chemical potential [23, 24] or McNabbFoster kinetic formulations [25–27]. The important role of mechanics is again evident in Eq. (10). In fact, in steady state (∂CL/∂t = 0), the hydrogen concentration in the lattice is given by,

with C0 being the reference concentration (e.g., the concentration intrinsic to the environment under consideration). This necessarily implies that the hydrogen content will be highest in regions of high σH and, therefore, that an accurate characterisation of crack tip stress fields over the critical distance for hydrogen-assisted cracking is of utmost importance.
A simple, back-of-the-envelope calculation can be used to determine at what distance ahead from the crack tip hydrogen-assisted damage takes place, exploiting the fact that hydrogen-assisted fracture exhibits a three-stage behaviour, as shown in Fig. 4a. After an initial stage where the crack growth rate grows with the applied K, a plateau is reached (stage II), where crack growth is insensitive to K as it is diffusion-controlled. Stage II cracking is intermittent as, for the crack to propagate, a sufficient amount of hydrogen must be attained at a critical distance ahead of the crack tip xcrit and the time to achieve this is governed by the diffusion of hydrogen from the crack tip surface. An approximate value of xcrit can be obtained by considering the measured crack speed and the time that it takes for a hydrogen atom to reach that critical distance xcrit. The latter can roughly be calculated through the analytical solution for 1D diffusion [28], whereby the diffusion distance d is defined to be a function of the diffusion coefficient and the time as d = 2√Dt (xcrit = 2√Dtcrit). Then, experimental measurements of da/dt (equivalent to xcrit/tcrit) and D can be used to estimate xcrit through the resulting simple system of two algebraic equations. For the Ni superalloy Monel K-500 investigated in Ref. [29], experiments reveal a diffusivity of D ≈ 0.01 µm2/s and a stage II da/dt ≈ 0.04 µm/s (as measured for the three replicate experiments at the cathodic potential of EAPP = −1000 mVSCE in Fig. 4b), rendering xcrit = 1 µm.
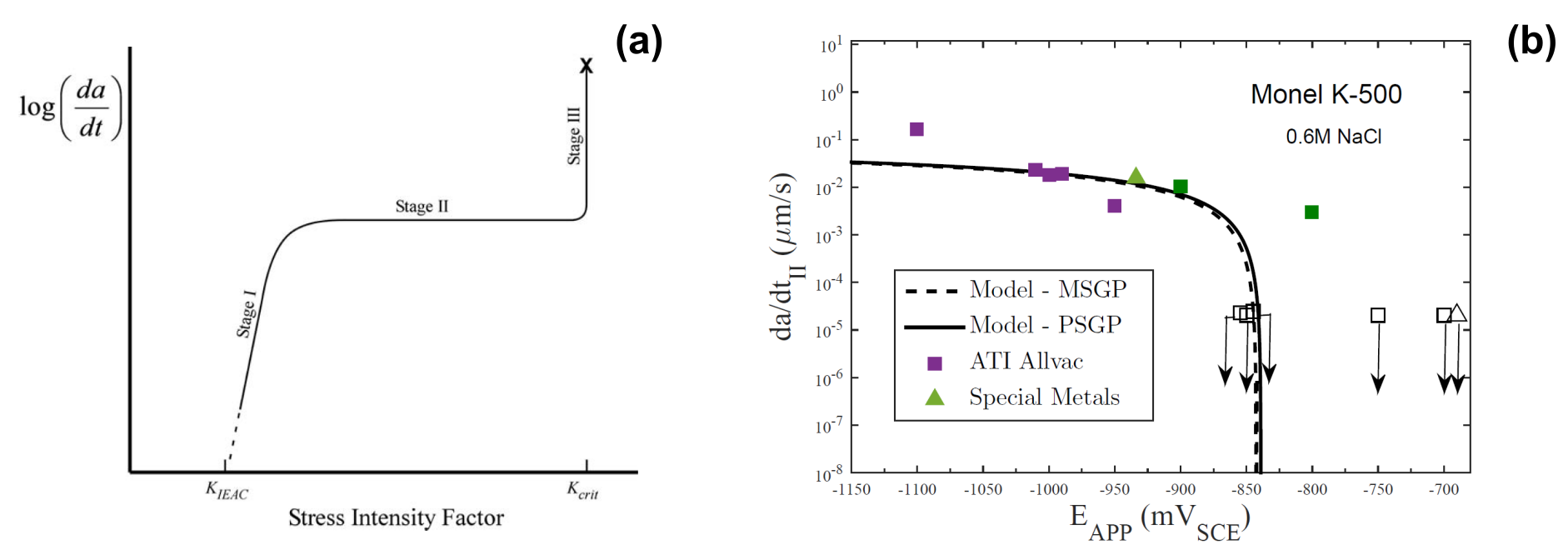
Figure 4: Determining the critical distance (xcrit) for hydrogen-assisted fracture by analysing the diffusion-controlled stage II crack growth behaviour; (a) typical stages involved in hydrogen-assisted crack growth (taken from Ref. [30]), and (b) experimental measurements and model predictions for stage II crack growth rates of Monel K-500 (as a function of the applied potential, EAPP) in a hydrogen-containing environment (taken from Ref. [29]).
The simple analysis above reveals the need to accurately characterise crack tip fields within microns ahead of the crack tip, where conventional continuum constitutive models break down. Arguably the main physical feature that conventional continuum theories fail to capture at a few microns ahead of a crack tip is the stress elevation associated with geometrically necessary dislocations (GNDs) [31, 32]. This flow stress elevation associated with plastic strain gradients and large dislocation densities has been consistently observed under similar conditions using indentation [33] or in a plethora of micro-scale experiments; from micro-torsion to constrained shear of thin films [34–36]. The plastic zone adjacent to the crack tip is physically small and contains large gradients of plastic strain, leading to local strengthening and a stress elevation that can have important implications for hydrogen embrittlement, given the exponential dependence of the hydrogen concentration on hydrostatic stresses and the central role that crack tip stresses play in triggering interface decohesion [37–40]. Fortunately, a number of mechanicians have been working on extending plasticity theory to account for these micro-scale hardening phenomena, through the development of so-called strain gradient plasticity theories [41–45]. These strain gradient plasticity models can capture the role that GNDs and plastic strain gradients play in elevating crack tip stresses and hydrogen concentrations, as highlighted in Fig. 5. Neglecting the dislocation phenomena that govern material behaviour at microns ahead of the crack tip significantly underpredicts the levels of tensile stress and hydrogen content that are attained at the critical distance of hydrogen-assisted cracking. The results shown in Fig. 5b reveal that the hydrogen content can readily be more than 20 times higher than that associated with the environment at 1 µm ahead of the crack tip due to local dislocation hardening mechanisms.

Figure 5: The role that plastic strain gradients and GNDs play on crack tip fields over the critical distance for hydrogen-assisted cracking. Predictions with conventional (von Mises) plasticity and a mechanism-based strain gradient plasticity theory [46] of (a) the tensile stress distribution ahead of the crack tip, and (b) the lattice hydrogen concentration, with C0 being the hydrogen concentration associated with the environment under consideration. Taken from Ref. [47]. For typical values relevant to hydrogen-assisted cracking (σY = 600 MPa, KIc = 35 MPa√m), the Irwin approximation of the plastic zone size implies that r/Rp = 0.003 at 1 µm ahead of the crack.
Underpredicting the dislocation density also has implications in the analysis of hydrogen trapping, as the dislocation density (and thus the hydrogen trapped at dislocations) increases significantly near cracks and other stress concentrators due to the GND contribution [47].
2.3 Hydrogen embrittlement
Once the uptake and local distribution of hydrogen have been quantified, it is time to connect these to the embrittlement process: the nucleation and growth of cracks as assisted by hydrogen. This requires a mechanistic interpretation of the interplay between relevant variables in the fracture process zone. A number of hydrogen embrittlement mechanisms have been proposed through the years. Arguably, the two most popular ones are the so-called Hydrogen-Enhanced Decohesion (HEDE) and Hydrogen-Enhanced Localised Plasticity (HELP) mechanisms.
HEDE postulates that hydrogen reduces the bonding strength between metal atoms, facilitating brittle decohesion [29, 48–50]. This is supported by experimental observations of brittle and cleavage-like features in the fracture surface and by atomistic calculations of surface energy reduction with increasing hydrogen coverage (see, e.g., Refs. [51–53]). However, critics argue that the reduction in atomic bonding strength (of 20-40%) is not sufficient to justify a shift from ductile to brittle behaviour, given that the lattice or grain boundary strengths are on the order of 9-10 times the yield stress and conventional plasticity analyses of crack tip stresses yield a maximum stress that is typically 3-4σY [54, 55]. Simulations accounting for GND effects provide a counter-argument to this as they result in much higher hydrogen contents (further reducing the bonding strength) and crack tip stresses (bringing them closer to the lattice strength). For example, at 1 µm ahead of the crack tip (the critical distance for embrittlement), the results from Fig. 5 show that tensile stresses are ∼ 8σY and that the hydrogen content is at least twice as large as that predicted using conventional continuum theories that neglect the role of plastic strain gradients. In other words, a strain gradient plasticity-based analysis of crack tip fields provides a modern rationalisation of hydrogen-enhanced decohesion mechanisms.
On the other hand, the HELP mechanism postulates that hydrogen embrittlement is the result of an increased mobility of dislocations due to hydrogen [56, 57]. This interpretation is supported by experimental observations of hydrogen-enhanced dislocation motion (see, e.g., [58]). However, critics argue that hydrogen-plasticity interactions appear to be modest, as shown (e.g.) by the tensile curves in Fig. 1a, where no difference in yield stress or hardening is observed. Moreover, it is unclear why an increased dislocation mobility will result in strain localisation and a macroscopic brittle response; in fact, an increased dislocation mobility is known to enhance ductility. Recently, Harris et al. [59] have shown that hydrogen embrittlement can take place in Ni even at cryogenic temperatures, where hydrogen-dislocation interactions are precluded.
Other interpretations that have been widely adopted include the adsorption-induced decohesion mechanism [60] and the hydrogen-enhanced strain-induced vacancy formation mechanism [61]. The latter postulates that the density and clustering of vacancies are promoted by the presence of hydrogen and that the voids resulting from vacancy coalescence will go on to cause failure through microvoid cracking mechanisms. While this mechanistic interpretation has been enjoying increasing popularity in recent years, it is unclear how the small voids that result from vacancy coalescence can drive fracture, particularly in the view of well-established size effects (smaller voids grow more slowly than bigger voids [62–64]). While under some environment-material combinations, small dimples are observed in the fracture surface (or just below it), this does not necessarily constitute an indication that cracking is through void coalescence (is only natural that voids emerge in areas of high plastic straining, yet fracture can be along cleavage planes or other brittle interfaces). In recent years, other interpretations have been postulated. For example, Vikram Deshpande and co-workers [65, 66] recently postulated a hydride-mediated ‘fast-fracture’ mechanism, whereby failure originates from the fast growth of cleavage cracks that initiate from cavities that form around inclusions such as carbide particles. On the other side, Bill Curtin and co-workers recently argued that hydrogen embrittlement is the result of atomic decohesion and the hindering of crack tip dislocation emission, which precludes crack tip blunting [67]. This reinvigorated search for theories is partially fuelled by the emergence of new experimental techniques, capable of achieving unprecedented resolution levels, such as Atom Probe Tomography [68, 69]. The field is in full swing.
3. Opportunities and challenges
The lack of mechanistic understanding underscores the need for further research and, particularly, for critical experiments that can shed new light on the interpretation of this longstanding challenge. Nevertheless, decarbonisation efforts require short-term solutions to address the pressing need to deploy infrastructure for hydrogen transport and storage. For example, Airbus has committed to operate the first zero-emission, hydrogen-based commercial aircraft by 2035. This constitutes a daunting challenge for engineers, not only they must handle an arguably unsolved problem but existing structural integrity protocols are no longer suitable. For example, fracture mechanics concepts such as the critical transition flaw size or KI vs KIc design, appropriate for homogeneous materials are not suited - hydrogen induces a spatial variation of the material toughness that challenges these approaches. This, however, constitutes an excellent opportunity for computational mechanicians, as Virtual Testing becomes more important than ever, and the interplay between deformation, hydrogen diffusion and fracture can be captured through suitable coupled finite element models (so-called multi-physics modelling).
Recent years have seen a surge in the development of coupled electro-chemo-mechanical models for hydrogen embrittlement. Among these, one approach that has been particularly popular is the combination of multi-physics modelling and phase field fracture [8, 70, 71]. Phase field fracture models [72] are particularly convenient as: (i) crack growth is predicted as an exchange between stored and fracture energy, (ii) they can handle cracking problems of arbitrary complexity, and (iii) the phase field equation can be readily coupled with equations describing other physical phenomena. A simple phase field-based model capable of capturing the interplay between deformation, diffusion and hydrogen-assisted fracture can be described with the following three balance equations:

where ϕ is the phase field variable, g(ϕ) is a degradation function that reduces the load-carrying capacity of the solid, C is the fourth-order material Jacobian, u is the displacement vector, ψ is the strain energy density (a function of the strain tensor ε), ℓ is the phase field length scale and Gc is the material toughness. A number of couplings are evident, most of which have been already discussed, but a key one emerges - how the toughness or fracture energy of the material varies with the hydrogen content: Gc (CL). In this regard, it should be noted that the framework is universal and can accommodate any mechanistic interpretation; Gc can be made a function of any relevant micro or macro-mechanical variable and phase field-based models for various mechanisms such as HEDE and HELP have been proposed [73, 74]. One possibility is to establish a connection with first principles, defining the variation of Gc based on atomistic calculations of surface energy variation with hydrogen coverage. This approach, inspired in the work by Michael Ortiz and co-workers with cohesive zone models [75], is illustrated in Fig. 7a, which shows DFT predictions of surface (fracture) energy as a function of the hydrogen content. The data, obtained for two types of grain boundaries in Ni, can be fit using a linear relationship, such that Gc = (1 − 0.41)θGc(0), where the occupancy of the relevant interface (in this case, a grain boundary) can be determined based on CL and Oriani’s equilibrium (Fig. 3). In this way, predictions can be delivered based purely on nominal material properties and one DFT calculation, and these seem to deliver a very good agreement with experiments [70].
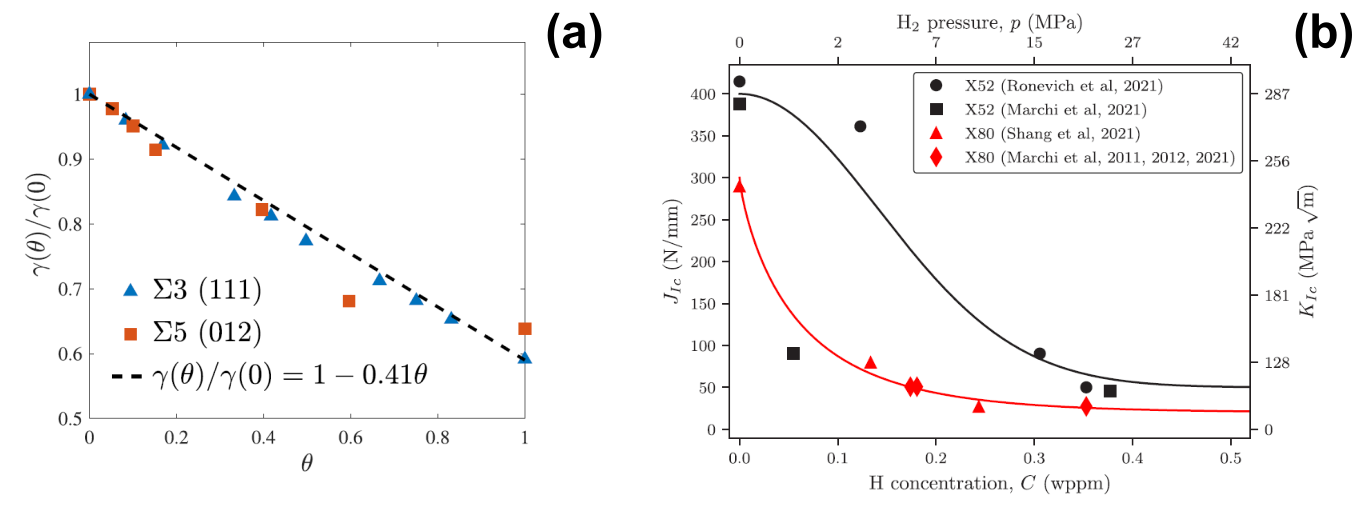
Figure 6: Approaches to quantifying the degradation of the material toughness with the hydrogen content: (a) mechanistic, based on atomistic calculations of surface energy dependency with hydrogen occupancy θ, and (b) phenomenological, based on the fitting of toughness versus concentration data. The former shows DFT data for two types of grain boundaries in Ni while the latter shows toughness versus hydrogen content data for two pipeline steels. Taken from Refs. [70, 76].
Another approach, more phenomenological, is to calibrate the hydrogen degradation function with experimental data on toughness versus hydrogen content. While this requires running fracture experiments over a sufficiently wide range of hydrogen concentrations, these data exist for most engineering materials. This class of computational models have been successfully used to reproduce experiments, gaining complementary insight, and predict the behaviour of large-scale engineering components, effectively enabling Virtual Testing in hydrogen-containing environments. Some examples are provided in Fig. 7, ranging from the prediction of laboratory experiments to determining what is the critical pressure at which hydrogen can be transported safely through existing natural gas pipeline infrastructure. For those who might be interested, more examples are also provided in the video below.

Figure 7: Example of applications of phase field-based deformation-diffusion-fracture finite element models, involving: (a) their use to predict the critical pressure at which hydrogen can be transported in existing pipeline infrastructure [76], accounting for the welding process, residual stresses and microstructural variations; (b) their application to large-scale engineering problems of high complexity, such as the failure of pipelines containing hundreds of pit corrosion defects or the fracture of bolts in concrete samples [77]; and (c) their use to predict fracture and fatigue laboratory experiments, showing their ability to capture data and replace costly experiments [70, 78].
While progress in computational and experimental hydrogen embrittlement research has been remarkable in recent years, there are still more questions than answers and the latter are needed urgently if we are to meet our decarbonisation goals. As is hopefully evident from this blog post, hydrogen embrittlement is very much a mechanics problem; mechanicians have a lot to say.
[Video] Towards a Virtual Hydrogen Lab
References
[1] W. H. Johnson, On some remarkable changes produced in iron and steel by the action of hydrogen and acids, Proceedings of the Royal Society of London 23 (1875) 168–179.
[2] R. P. Gangloff, Hydrogen-assisted Cracking, Elsevier Science, 2003, pp. 31–101.
[3] R. P. Gangloff, B. P. Somerday, Gaseous Hydrogen Embrittlement of Materials in Energy Technologies, Woodhead Publishing Limited, 2012.
[4] Z. S. Hosseini, M. Dadfarnia, K. A. Nibur, B. P. Somerday, R. P. Gangloff, P. Sofronis, Trapping against hydrogen embrittlement, ASME Press, 2017, pp. 71–80.
[5] T. Iwadate, T. Karaushi, J. Watanabe, Prediction of fracture toughness k ic of 21/4cr-1mo pressure steels from charpy v-notch test results, in: Flaw Growth and Fracture, ASTM International, 1977.
[6] J. A. Ronevich, Fatigue performance of high-strength pipeline steels and their welds in hydrogen gas service., Tech. rep., Sandia National Lab.(SNL-NM), Albuquerque, NM (United States) (2018).
[7] K. Xu, M. Rana, Tensile and fracture properties of carbon and low alloy steels in high pressure hydrogen, in: Proceedings of the 2008 international hydrogen conference, 2009, pp. 349–356.
[8] L. Anand, Y. Mao, B. Talamini, On modeling fracture of ferritic steels due to hydrogen embrittlement, Journal of the Mechanics and Physics of Solids 122 (2019) 280–314.
[9] O. Barrera, D. Bombac, Y. Chen, T. D. Daff, E. Galindo-Nava, P. Gong, D. Haley, R. Horton, I. Katzarov, J. R. Kermode, C. Liverani, M. Stopher, F. Sweeney, Understanding and mitigating hydrogen embrittlement of steels: a review of experimental, modelling and design progress from atomistic to continuum, Journal of Materials Science 53 (2018) 6251–6290
[10] M. B. Djukic, G. M. Bakic, V. S. Zeravcic, A. Sedmak, B. Rajicic, The synergistic action and interplay of hydrogen embrittlement mechanisms in steels and iron: Localized plasticity and decohesion, Engineering Fracture Mechanics 216 (2019) 106528.
[11] T. Paxton, A. P. Sutton, M. W. Finnis, The challenges of hydrogen and metals, Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 375 (2017).
[12] T. Hageman, E. Martinez-Paneda, An electro-chemo-mechanical framework for predicting hydrogen uptake in metals due to aqueous electrolytes, Corrosion Science 208 (2022) 110681.
[13] T. Hageman, E. Martinez-Paneda, Stabilising effects of lumped integration schemes for the simulation of metal-electrolyte reactions, Journal of the Electrochemical Society 170 (2023) 021511.
[14] T. Hageman, E. Martınez-Paneda, A phase field-based framework for electro-chemomechanical fracture: Crack-contained electrolytes, chemical reactions and stabilisation, Computer Methods in Applied Mechanics and Engineering 415 (2023) 116235.
[15] C. S. Marchi, B. P. Somerday, S. L. Robinson, Permeability, solubility and diffusivity of hydrogen isotopes in stainless steels at high gas pressures, International Journal of Hydrogen Energy 32 (2007) 100–116.
[16] A. Díaz, J. M. Alegre, I. I. Cuesta, Coupled hydrogen diffusion simulation using a heat transfer analogy, International Journal of Mechanical Sciences 115-116 (2016) 360–369.
[17] D. Li, R. P. Gangloff, J. R. Scully, Hydrogen trap states in ultrahigh-strength aermet 100 steel, Metallurgical and Materials Transactions A: Physical Metallurgy and Materials Science 35 A (2004) 849–864.
[18] A. Turnbull, Perspectives on hydrogen uptake, diffusion and trapping, International Journal of Hydrogen Energy 40 (2015) 16961–16970.
[19] R. Fernández-Sousa, C. Betegón, E. Martinez-Paneda, Analysis of the influence of microstructural traps on hydrogen assisted fatigue, Acta Materialia 199 (2020) 253–263
[20] R. A. Oriani, P. H. Josephic, Equilibrium aspects of hydrogen induced cracking of steels, Acta Metallurgica 22 (1974) 1065–1074.
[21] C. Ayas, V. S. Deshpande, N. A. Fleck, A fracture criterion for the notch strength of high strength steels in the presence of hydrogen, Journal of the Mechanics and Physics of Solids 63 (2014) 80–93.
[22] P. Sofronis, R. M. McMeeking, Numerical analysis of hydrogen transport near a blunting crack tip, Journal of the Mechanics and Physics of Solids 37 (1989) 317–350.
[23] C. V. D. Leo, L. Anand, Hydrogen in metals: A coupled theory for species diffusion and large elastic-plastic deformations, International Journal of Plasticity 43 (2013) 42–69.
[24] E. Elmukashfi, E. Tarleton, A. C. F. Cocks, A modelling framework for coupled hydrogen diffusion and mechanical behaviour of engineering components, Computational Mechanics 66 (2020) 189–220.
[25] E. Martınez-Paneda, A. Díaz, L. Wright, A. Turnbull, Generalised boundary conditions for hydrogen transport at crack tips, Corrosion Science 173 (2020) 108698.
[26] E. Legrand, A. Oudriss, C. Savall, J. Bouhattate, X. Feaugas, Towards a better understanding of hydrogen measurements obtained by thermal desorption spectroscopy using fem modeling, International Journal of Hydrogen Energy 40 (2015) 2871–2881.
[27] Y. Charles, J. Mougenot, M. Gasperini, Effect of transient trapping on hydrogen transport near a blunting crack tip, International Journal of Hydrogen Energy 46 (18) (2021) 10995–11003.
[28] J. Crank, The mathematics of diffusion, Oxford University Press, 1979.
[29] E. Martinez-Paneda, C. F. Niordson, R. P. Gangloff, Strain gradient plasticity-based modeling of hydrogen environment assisted cracking, Acta Materialia 117 (2016) 321–332.
[30] T. L. Anderson, Fracture Mechanics. Fundamentals and Applications, 3rd Edition, CRC Press, Taylor & Francis, 2005
[31] U. Komaragiri, S. R. Agnew, R. P. Gangloff, M. R. Begley, The role of macroscopic hardening and individual length-scales on crack tip stress elevation from phenomenological strain gradient plasticity, Journal of the Mechanics and Physics of Solids 56 (2008) 3527–3540.
[32] E. Martínez-Paneda, C. F. Niordson, On fracture in finite strain gradient plasticity, International Journal of Plasticity 80 (2016) 154–167.
[33] W. D. Nix, H. J. Gao, Indentation size effects in crystalline materials: A law for strain gradient plasticity, Journal of the Mechanics and Physics of Solids 46 (1998) 411–425.
[34] N. A. Fleck, G. M. Muller, M. F. Ashby, J. W. Hutchinson, Strain gradient plasticity: Theory and experiment, Acta Metallurgica et Materialia 42 (1994) 475–487.
[35] J. S. Stolken, A. G. Evans, A microbend test method for measuring the plasticity length scale, Acta Materialia 46 (1998) 5109–5115.
[36] Y. Mu, J. Hutchinson, W. Meng, Micro-pillar measurements of plasticity in confined cu thin films, Extreme Mechanics Letters 1 (2014) 62–69.
[37] Y. Wei, J. W. Hutchinson, Steady-state crack growth and work of fracture for solids characterized by strain gradient plasticity, Journal of the Mechanics and Physics of Solids 45 (1997) 1253–1273.
[38] Y. Wei, G. Xu, A multiscale model for the ductile fracture of crystalline materials, International Journal of Plasticity 21 (2005) 2123–2149.
[39] E. Martínez-Paneda, C. Betegón, Modeling damage and fracture within strain-gradient plasticity, International Journal of Solids and Structures 59 (2015) 208–215.
[40] E. Martínez-Paneda, V. S. Deshpande, C. F. Niordson, N. A. Fleck, The role of plastic strain gradients in the crack growth resistance of metals, Journal of the Mechanics and Physics of Solids 126 (2019) 136–150.
[41] N. A. Fleck, J. W. Hutchinson, A reformulation of strain gradient plasticity, Journal of the Mechanics and Physics of Solids 49 (2001) 2245–2271.
[42] P. Gudmundson, A unified treatment of strain gradient plasticity, Journal of the Mechanics and Physics of Solids 52 (2004) 1379–1406.
[43] M. E. Gurtin, L. Anand, A theory of strain-gradient plasticity for isotropic, plastically irrotational materials. part i: Small deformations, Journal of the Mechanics and Physics of Solids 53 (2005) 1624–1649.
[44] G. Z. Voyiadjis, Y. Song, Strain gradient continuum plasticity theories: Theoretical, numerical and experimental investigations, International Journal of Plasticity 121 (2019) 21–75.
[45] A. Panteghini, L. Bardella, C. F. Niordson, A potential for higher-order phenomenological strain gradient plasticity to predict reliable reponse under non-proportional loading, Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences 475 (2019) 20190258.
[46] H. Gao, Y. Hang, W. D. Nix, J. W. Hutchinson, Mechanism-based strain gradient plasticity - i. theory, Journal of the Mechanics and Physics of Solids 47 (1999) 1239–1263.
[47] M. Isfandbod, E. Martínez-Paneda, A mechanism-based multi-trap phase field model for hydrogen assisted fracture, International Journal of Plasticity 144 (2021) 103044.
[48] A. R. Troiano, The role of hydrogen and other interstitials in the mechanical behavior of metals: (1959 edward de mille campbell memorial lecture), Metallography, Microstructure, and Analysis 5 (2016) 557–569.
[49] R. A. Oriani, A mechanistic theory of hydrogen embrittlement of steels, Berichte der Bunsengesellschaft f¨ur physikalische Chemie 76 (1972) 848–857.
[50] W. W. Gerberich, R. A. Oriani, M. Lii, X. Chen, T. Foecke, The necessity of both plasticity and brittleness in the fracture thresholds of iron, Philosophical Magazine A 63 (1991) 363–376.
[51] A. Alvaro, I. T. Jensen, N. Kheradmand, O. M. Løvvik, V. Olden, Hydrogen embrittlement in nickel, visited by first principles modeling, cohesive zone simulation and nanomechanical testing, International Journal of Hydrogen Energy 40 (2015) 16892–16900.
[52] D. E. Jiang, E. A. Carter, Diffusion of interstitial hydrogen into and through bcc fe from first principles, Physical Review B - Condensed Matter and Materials Physics 70 (2004) 1–9.
[53] A. Tehranchi, W. Curtin, Atomistic study of hydrogen embrittlement of grain boundaries in nickel: I. fracture, Journal of the Mechanics and Physics of Solids 101 (2017) 150–165.
[54] J. R. Rice, E. P. Sorensen, Continuing crack-tip deformation and fracture for plane-strain crack growth in elastic-plastic solids, Journal of the Mechanics and Physics of Solids 26 (1978) 163–186.
[55] V. Tvergaard, J. W. Hutchinson, The relation between crack growth resistance and fracture process parameters in elastic-plastic solids, Journal of the Mechanics and Physics of Solids 40 (1992) 1377–1397.
[56] H. K. Birnbaum, P. Sofronis, Hydrogen-enhanced localized plasticity - a mechanism for hydrogen related fracture, Materials Science and Engineering A 176 (1994) 191–202.
[57] I. M. Robertson, P. Sofronis, A. Nagao, M. L. Martin, S. Wang, D. W. Gross, K. E. Nygren, Hydrogen embrittlement understood, Metallurgical and Materials Transactions B 46 (2015) 1085–1103.
[58] L. Huang, D. Chen, D. Xie, S. Li, Y. Zhang, T. Zhu, D. Raabe, E. Ma, J. Li, Z. Shan, Quantitative tests revealing hydrogen-enhanced dislocation motion in α-iron, Nature Materials 22 (6) (2023) 710–716.
[59] Z. D. Harris, S. K. Lawrence, D. L. Medlin, G. Guetard, J. T. Burns, B. P. Somerday, Elucidating the contribution of mobile hydrogen-deformation interactions to hydrogen-induced intergranular cracking in polycrystalline nickel, Acta Materialia 158 (2018) 180–192.
[60] S. Lynch, Discussion of some recent literature on hydrogen-embrittlement mechanisms: Addressing common misunderstandings, Corrosion Reviews 37 (2019) 377–395.
[61] M. Nagumo, Hydrogen related failure of steels–a new aspect, Materials Science and Technology 20 (8) (2004) 940–950.
[62] J. Hutchinson, N. Fleck, Strain gradient plasticity, Adv. Appl. Mech 33 (1997) 295–361.
[63] Y. Huang, H. Gao, W. Nix, J. Hutchinson, Mechanism-based strain gradient plasticity—ii.analysis, Journal of the Mechanics and Physics of Solids 48 (1) (2000) 99–128.
[64] B. Liu, X. Qiu, Y. Huang, K. Hwang, M. Li, C. Liu, The size effect on void growth in ductile materials, Journal of the Mechanics and Physics of Solids 51 (7) (2003) 1171–1187.
[65] S. S. Shishvan, G. Csanyi, V. S. Deshpande, Hydrogen induced fast-fracture, Journal of the Mechanics and Physics of Solids 134 (2020) 103740.
[66] S. Shishvan, G. Csanyi, V. Deshpande, Strain rate sensitivity of the hydrogen embrittlement of ferritic steels, Acta Materialia 257 (2023) 119173.
[67] X. Zhou, A. Tehranchi, W. A. Curtin, Mechanism and prediction of hydrogen embrittlement in fcc stainless steels and high entropy alloys, Physical review letters 127 (17) (2021) 175501.
[68] Y.-S. Chen, D. Haley, S. S. A. Gerstl, A. J. London, F. Sweeney, R. A. Wepf, W. M. Rainforth, P. A. J. Bagot, M. P. Moody, Direct observation of individual hydrogen atoms at trapping sites in a ferritic steel, Science 355 (2017) 1196–1199.
[69] Y.-S. Chen, H. Lu, J. Liang, A. Rosenthai, H. Liu, G. Sneddon, I. McCarroll, Z. Zhao, W. Li, A. Guo, J. M. Cairney, Observation of hydrogen trapping at dislocations, grain boundaries, and precipitates, Science 175 (2020) 171–175.
[70] E. Martínez-Paneda, A. Golahmar, C. F. Niordson, A phase field formulation for hydrogen assisted cracking, Computer Methods in Applied Mechanics and Engineering 342 (2018) 742–761.
[71] F. P. Duda, A. Ciarbonetti, S. Toro, A. E. Huespe, A phase-field model for solute-assisted brittle fracture in elastic-plastic solids, International Journal of Plasticity 102 (2018) 16–40.
[72] B. Bourdin, G. A. Francfort, J. J. Marigo, The variational approach to fracture, Springer Netherlands, 2008.
[73] P. K. Kristensen, C. F. Niordson, E. Martínez-Paneda, A phase field model for elasticgradient-plastic solids undergoing hydrogen embrittlement, Journal of the Mechanics and Physics of Solids 143 (2020) 104093.
[74] C. Huang, X. Gao, Phase field modeling of hydrogen embrittlement, International Journal of Hydrogen Energy 45 (2020) 20053–20068.
[75] S. Serebrinsky, E. A. Carter, M. Ortiz, A quantum-mechanically informed continuum model of hydrogen embrittlement, Journal of the Mechanics and Physics of Solids 52 (2004) 2403–2430.
[76] T. K. Mandal, J. Parker, M. Gagliano, E. Martínez-Paneda, Computational predictions of weld structural integrity in hydrogen transport pipelines, International Journal of Hydrogen Energy (1 2024). doi:10.1016/j.ijhydene.2024.01.258.
[77] P. K. Kristensen, C. F. Niordson, E. Martínez-Paneda, Applications of phase field fracture in modelling hydrogen assisted failures, Theoretical and Applied Fracture Mechanics 110 (2020) 102837.
[78] C. Cui, P. Bortot, M. Ortoloni, E. Martínez-Paneda, Computational predictions of hydrogen-assisted fatigue crack growth (2024).
- Emilio Martínez Pañeda's blog
- Log in or register to post comments
- 2103 reads


Recent comments